HPV Vaccines: Separating Truth from Fiction
Recently, I’ve received a lot of requests on social media to discuss HPV vaccines specifically, and I thought I could be brief enough about it that I would not require a blog post, but apparently not because it turns out there’s a lot to cover. I’ve always been a bit puzzled by the response to HPV vaccines because of how frequently I hear something to the effect of “I feel really good about all vaccines- except that one.” So, let’s look at the evidence together.
The Fundamentals: HPV and its vaccines
Before discussing HPV vaccines, we should get oriented. Let’s go through some key facts.
Plotkin SA, Orenstein W, Offit DPA, Edwards KM. 2017. Plotkin’s Vaccines. 7th ed. Elsevier. Figure 30.7
Human papillomavirus (HPV) refers to over 200 strains of small, double-stranded DNA viruses in the Papillomaviridae genus. Some (but not all) of these strains are particularly important for human health because they can cause tumor formation and result in the development of cancer, in particular: cervical, ovarian, vulvovaginal, penile, scrotal, anal, and oropharyngeal cancers. In addition, HPV can also cause the formation of benign but psychologically distressing warts (papillomas) at affected sites. HPV is the most common sexually transmitted infection and is commonly contracted shortly after the first sexual contact (sometimes known as “sexual debut” in the literature).
Infection and its consequences cannot be perfectly protected against via barrier methods (i.e. condoms) because tissues containing lesions with the virus may still make contact from person to person. Transmission occurs through intimate skin-to-skin contact, typically through vaginal, penile, anal, or oral sex. Globally, about 1.01 million cases of cancer due to HPV occur each year (you can view the cases in the US here). Autoinoculation (self-infection) of one site on the body to another by transferring virus can occur. HPV can cause tumors in both immunocompromised and immunocompetent patients. HPV strains are extremely specific and as a result, infection with one type does not confer protection against other types (though in vaccine trials there was some small protective effect against non-covered strains of HPV from the Gardasil vaccine; the effect was larger from Cervarix). The incubation period for the virus is usually about 3 months, but disease can become apparent in weeks to years. Tumor formation typically occurs over a decade. Many HPV lesions will spontaneously resolve in a period of 1-2 years, but predicting in whom remains a challenge as even in otherwise healthy people, persistent infection (and therefore warts and cancer) may occur.
Current HPV vaccines (i.e. Gardasil 9) are recommended in females aged 9 to 45 for the prevention of cervical, vulvar, vaginal, anal, oropharyngeal, and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58 and genital warts (condyloma acuminata) caused by HPV types 6 and 11, as well as any precancerous lesions resulting from these viruses. They are additionally recommended for use in males aged 9 to 45 for the prevention of anal, oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58, genital warts (condyloma acuminata) caused by HPV types 6 and 11, and any precancerous lesions caused by those viral infections.
An HPV virus-like particle as visualized by cryoelectron microscopy. The outer structures are the L1 protein of HPV. This is made from 360 copies of the protein. The inside of the particle is empty.
Smith DM, Simon JK, Baker JR Jr. 2013. Applications of nanotechnology for immunology. Nat Rev Immunol. 13(8):592–605.
HPV vaccines are all composed of virus-like particles (VLPs; with 360 proteins on the surface) made of the HPV L1 proteins with an aluminum salt adjuvant. Currently, the only one on the market is Gardasil-9, which protects against 9 of the strains of HPV most likely to cause cancer and other serious health problems (e.g. recurrent respiratory papillomatosis). The proteins from HPV are made inside yeast (anyone who has a history of immediate allergic reactions to yeast should not receive these vaccines), which has DNA encoding the L1 proteins from different strains of HPV, that then spontaneously self-assemble into a spherical structure that superficially resembles a virus. Normally, the L1 proteins allow the virus to enter cells, but on their own don’t do much. In other words, the particle can’t harm you.
The principle behind the action of the vaccines is thought to be the result of antibodies that block entry of HPV into the cell. In other words, HPV vaccines are truly special in that they actually are able to prevent infection (i.e. inducing sterilizing immunity) rather than just effectively prime the immune system against HPV to rapidly clear infection before disease can occur (which is how most vaccines work). It’s worth noting however that this is possible only because of a few things:
A BPV (bovine papillomavirus- a relative of human papillomaviruses) visualized by cryoelectron microscopy, with DNA underneath reconstructed in (B). Note the similarity to the virus-like particle. From Buck CB, Cheng N, Thompson CD, et al. Arrangement of L2 within the papillomavirus capsid. J Virol 2008;82:5190–5197
HPV takes advantage of a phenomenon called immunological ignorance- for mechanisms that aren’t entirely clear, HPV antigens alone are not very good at inducing T cell responses that are needed to clear the infection once it has occurred. This is related to some intrinsic properties of an antigen-presenting cell called a Langerhans cell, found throughout the skin and in the female genital tract.
HPV infection into a cell is an incredibly slow process that takes hours. Most viruses can get into a cell within a matter of seconds. This means that there is a huge window of time for antibodies to be effective in stopping the virus, which is not going to be true of most infections.
The genital mucosa of the female reproductive tract has some unique immunological properties; specifically, there are high levels of IgG antibodies secreted into the tract which is unusual (mucosal sites are ordinarily enriched in IgA rather than IgG, and thus effective vaccines for mucosal infections have to elicit IgA at the site of infection, which is a significant challenge).
HPV requires microscopic injuries to the skin (i.e. abrasions) to be able to enter target cells and cause infection, but these injuries also provide an opportunity for antibodies to exit through the microscopic vasculature and neutralize HPV (bind it and prevent it from getting into the cell). This is thought to be the major mechanism by which the vaccines are protective.
These things taken together mean that sterilizing immunity against HPV is actually somewhat easy to accomplish (well, once you know how to make effective vaccines at least). In fact, antibodies from vaccinated individuals can be diluted 1000 times and still effectively prevent HPV infection, and their decay following vaccination is quite slow. This suggests that immunity to HPV conferred by vaccination is likely to last for a very long time, potentially lifelong but certainly at least 10 years. In other words, HPV-induced cancers and disease are uniquely preventable, which is amazing to think about. Australia is in fact poised to eliminate cervical cancer completely, potentially by 2028.
This technology isn’t new- it is in fact how hepatitis B vaccines are made, which use virus-like particles composed of the hepatitis B surface antigen (HBSAg) which are adsorbed onto an aluminum salt; these vaccines have been available since 1965. As for HPV vaccines, they have been in clinical trials since 1997 (that’s the year I was born) and have been publicly available since 2006. In short, I don’t think anyone can reasonably argue that these vaccines represent something extremely novel or untested. In fact, HPV vaccines might be among our most thoroughly examined vaccines on the market (with the possible exception of COVID-19 vaccines).
What controversies exist about the use of HPV vaccines?
There is apparently a belief among some people that HPV vaccines are controversial vaccines. I’m not sure where this idea came from, but it’s just not true. One really good way to tell whether or not a true controversy exists in a field -meaning that there are reasonable experts who stand at opposite sides of the issue- is to examine the recommendations of expert bodies regarding that topic. For example, the American College of Obstetricians and Gynecologist has a committee opinion on the use of HPV vaccines:
The Advisory Committee on Immunization Practices and ACOG recommend routine human papillomavirus (HPV) vaccination for girls and boys at the target age of 11–12 years (but it may be given from the age of 9 years) as part of the adolescent immunization platform.
As does the Advisory Committee on Immunization Practices:
HPV vaccine is recommended for routine vaccination at age 11 or 12 years (1). ACIP also recommends vaccination for females aged 13 through 26 years and males aged 13 through 21 years not vaccinated previously. Vaccination is also recommended through age 26 years for men who have sex with men and for immunocompromised persons (including those with HIV infection) if not vaccinated previously (1)
As does the WHO:
WHO recommends the HPV vaccine for girls in the age group of 9–13 years. Girls receiving a first dose of HPV vaccine before the age of 15 years can use a two-dose schedule. The interval between the two doses should be six months. There is no maximum interval between the two doses; however, an interval of no greater than 12–15 months is suggested. If the interval between doses is shorter than five months, then a third dose should be given at least six months after the first dose. Immunocompromised individuals, including those who are living with HIV, and females aged 15 years and older should also receive the vaccine and need three doses (at 0, 1–2, and 6 months schedule) to be fully protected.
As does the AAP:
The AAP recommends beginning the HPV vaccination series between 9 and 12 years of age, at an age that the provider deems optimal for acceptance. This earlier immunization age enhances the potential completion of the vaccination series and provides earlier protection, which could result in fewer cancer incidences.
And so on.
The attempt to manufacture controversy is a very well documented method among people who oppose scientific findings to manipulate public sentiment and action (read Merchants of Doubt for detailed examples). In essence, it does not necessarily even take active controversy to manipulate public sentiment- merely suggesting that the recommendations of expert bodies are not definitive or certain is enough to forestall policy action and result in inaction among the public. But in this case that inaction means preventable cases of cancer and warts.
That said, clearly some in the public do have some concerns, so it’s worth considering their validity.
Celentano DD, Szklo M, Gordis L. 2018. Gordis Epidemiology. 6th ed. Elsevier.
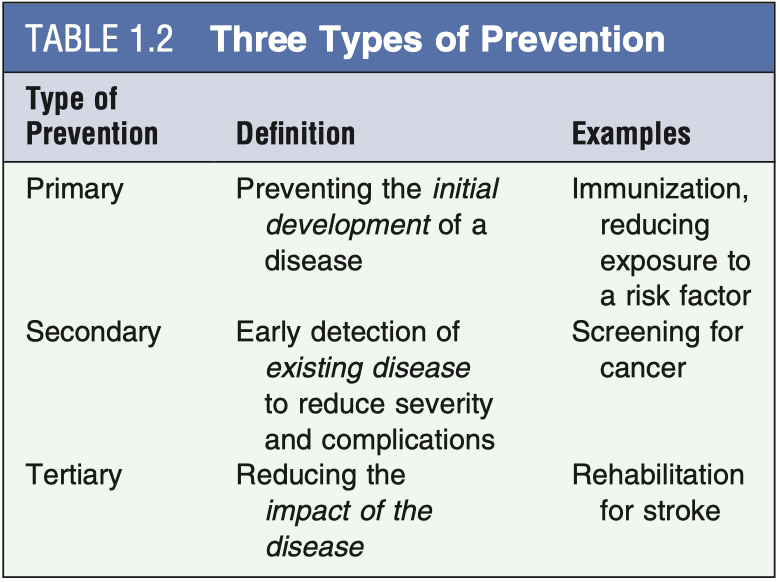
If HPV vaccines are so effective, why do vaccinated people still need Pap smears?
These are intended for different things. Vaccination against HPV is primary prevention- it is intended to stop you from even getting infected with HPV, thereby preventing you from developing precancerous lesions and eventually cancer. Screening with Pap tests is a form of secondary prevention. It is intended to detect the presence of a lesion that is already there so that it can be intervened upon before it progresses. HPV vaccines are approximately 97% effective at preventing HPV infection, and the 9-strain vaccine covers approximately 90% of all those HPV strains which cause cervical cancer. But that still means that there are some types of HPV which can cause cervical cancer that are not covered by the vaccine (though rare) and a small portion of people may not initiate effective responses against HPV through vaccination. Because of this, pap smears are still required- at least until we can eliminate cervical cancer completely.
Beyond this, HPV causes a number of other cancers which are not necessarily screened for routinely in the manner that cervical cancer is e.g. vulvovaginal, penile, oropharyngeal, etc.
If you’re wondering about Diane Harper here, check this explanation out from Dr. Vince Iannelli.
Why do males also need the HPV vaccine?
Males have tissues which can also be harmed by HPV infection, and by extension so can the bodies attached to those tissues. They can also get genital warts, as well as develop penile, anal, and oropharyngeal cancers. In addition, vaccinating them gets us closer to herd immunity against HPV because it prevents them from giving it to a partner. Initially, there were some countries which did not adopt a sex-neutral recommendation for HPV vaccination, but this was based around considerations of cost at the public health level rather than a lack of benefit for them. The vaccine HAS been tested in males.
How was the HPV vaccine studied before it was licensed? Was it compared against placebo?
Plotkin SA, Orenstein W, Offit DPA, Edwards KM. 2017. Plotkin’s Vaccines. 7th ed. Elsevier. Table 30.3
Studying the HPV vaccines is a bit more complicated than might be suggested by first blush. HPV vaccines aren’t given so much to prevent HPV as much as to prevent cancer, in particular cervical cancer. But this complicates measuring their efficacy. For example, it would never be appropriate to use the development of cervical cancer as an endpoint to judge the efficacy of the vaccines. That’s because:
It takes a decade or more for HPV infection to develop into cancer. The trials would have been endless to accumulate enough events that a judgment of efficacy to be made. Furthermore, cervical cancer from HPV progresses in discrete stages, and as the vaccine is prophylactic- intending to prevent that from happening- it could be considered a failure if a precancerous lesion corresponding to a covered HPV strain (e.g. CIN-1/2/3) appeared in a vaccinated individual.
The subject pool you screen cannot be allowed to progress into full blown cervical cancer if precancerous lesions are found. Hence this is absolutely not an appropriate way to judge HPV vaccine efficacy pre-licensure. After guidance from experts at the WHO, FDA, and EMA, Merck and GlaxoSmithKline (GSK- manufacturer of the Cervarix vaccine, which is very similar to Gardasil but used a slightly different adjuvant) set the development of CIN 2+ as the endpoint (a precancerous lesion which normally triggers ablative therapy).
By every reasonable definition of the term “placebo” the answer to the second question is “yes,” but let’s clarify. “Placebo” is defined by Portney’s Foundations of Clinical Research: Applications to Evidence-Based Medicine 4th Edition as:
A control that is similar in every way to the experimental treatment except that it does not contain the active component that comprises the intervention’s actions.
For a vaccine, under this definition, the placebo must be everything except for the vaccine antigen- in this case the HPV VLP. This is how Gardasil’s pre-licensure trials were designed: there was a control group which received only the vaccine adjuvant; note that there were also trials in which patients received saline placebo however. There are some ethical complications here, however. There’s an important concept in clinical research known as standard of care- if there is an established therapy or preventive measure for a given condition, and you are trialing something experimental for the same condition, you are not permitted to withhold that treatment of known efficacy from your placebo group. Consider for example a trial evaluating a medication for patients experiencing heart attacks. It would never be okay to withhold nitroglycerin, antiplatelets, beta blockers, and statins (for example) from the placebo group and just allow them to die of heart attacks- instead these patients would get all of these treatments in the trial, collectively serving as an active comparator arm. For that reason, Gardasil-9 was compared with the original quadrivalent (4-strain) Gardasil, where it had to show protection against the strains covered by Gardasil to be at least as good, in addition to protecting against 5 additional strains. It would not have been ethical to give a placebo in the common sense for these prelicensure trials.
With vaccines there’s another issue, however. In addition to the ethical aspects, it is important to preserve blinding, and vaccines are all associated with certain responses from the immune system that can feel uncomfortable, which are collectively termed “reactogenicity.” Using pure saline is unlikely to reproduce these reactogenicity effects and thus can bias participant behavior, for instance by making them more cautious and less likely to be exposed to the disease in question and thus understating the efficacy of the vaccine. For that reason, Cervarix, the other HPV vaccine that is no longer offered in the US, was compared against the hepatitis A vaccine, which contains inactivated hepatitis A virus with an aluminum adjuvant.
Review of the pre-licensure clinical trial data did not reveal any significant safety concerns (page 6).
Guo F, Cofie LE, Berenson AB. 2018. Cervical cancer incidence in young U.s. females after human Papillomavirus vaccine introduction. Am J Prev Med. 55(2):197–204.
Has the HPV vaccine actually been shown to prevent cervical cancer?
The answer here is an unqualified yes. As far as I can tell, this question stems from the choice in clinical trials to measure the ability of the vaccine to prevent CIN2 or higher grade cervical lesions rather than the development of cervical cancer itself. As above, this was based on ethical and practical considerations. To reiterate, it is absolutely appropriate to use the development of precancerous lesions to judge whether or not an intervention effectively prevents cancer for some interval. A cancerous tumor does not sprout instantly- it has to progress in stages from the precancerous lesions which are very well defined for many cancers, including cervical cancer. The development of those lesions in the treatment group indicates a failure of the intervention, in this case, HPV vaccination- but this occurrence is extremely rare. However, the HPV vaccine has been out for quite a while now, and we do have data showing that indeed, it does prevent cervical cancer. The efficacy of the HPV vaccine is extremely impressive- the FUTURE II trial followed over 2000 women who were vaccinated against HPV and found NO cases of cervical cancer among them in a period of 12 years.
Why do we need an HPV vaccine if 90% of infections clear up on their own?
The fact that 90% of infections clear up on their own means that 10% of them don’t. HPV is the most common sexually transmitted infection, to the extent that it is almost inevitable that anyone who is sexually active will contract it at some point in their lifetime. Globally, HPV is estimated to cause over 1 million cases of cancer each year, with over 45,000 cases in the US. That is a massive public health problem.
My child isn’t having sex. Why do they need this vaccine?
That’s kind of the point. HPV infection generally occurs near the time of the first sexual encounter (sexual debut). Effective prevention requires that they develop immunity before that encounter so that they don’t develop any HPV-associated lesions or cancer. There is also the additional benefit that vaccinating children at this age produces higher antibody titers, and they need only 2 doses.
Does the HPV vaccine make adolescents more sexually promiscuous?
No. This has been examined here, here, here, and here and in no study has there been a meaningful increase in sexually promiscuous behavior among vaccinees (that I could find anyway). But I do think there’s an important thought experiment attached to this question for those who are particularly concerned by it: whatever taboos are attached to sex for you, is your child getting cancer truly an appropriate punishment for violating them?
Do HPV vaccines cause infertility?
Put simply, no.
There’s a fairly extensive odyssey at work here behind this claim, and in the interest of brevity, I will hit upon the highlights only. Part of the claim seems to stem from this idea that because HPV vaccines prevent HPV, they can provoke an immune response in the reproductive organs and compromise their function, but this really isn’t how the vaccine works. If this were true, the vaccine could be expected to have value therapeutically (as it could stimulate the immune system to clear extant HPV lesions by killing infected cells), but therapeutic HPV vaccines are still a ways away. Then seemingly the claim became that HPV vaccines are a cause of primary ovarian insufficiency (POI). This too has been examined: HPV vaccination is not associated with an increased risk of POI (in fact, the study actually finds that the risk of POI among those being vaccinated is 70% lower than in unvaccinated individuals, but because of the rarity of the condition and therefore the small number of cases, the effect is explainable by random chance). Then apparently Gayle DeLong, an individual with a history of espousing anti-vaccine disinformation and no relevant credentials to the field of vaccine science, attempted to publish a now-retracted study claiming that females aged 25-29 who had received HPV vaccines were less likely to have conceived a child compared with those who had not. When a study is retracted, it indicates something truly egregious is wrong with it. What was wrong with DeLong’s work? By far the most serious problem with the manuscript is the fact that survey participants were asked about their use of birth control but these findings were not reported in the manuscript. This goes beyond an error- this is essentially overt fraud. On top of this, the work had a litany of statistical issues and failed to account for important confounders e.g. women who were vaccinated tended to have higher levels of educational attainment and income and would be more likely to defer having children to a later age compared with their unvaccinated cohort. But, if we’re claiming that HPV vaccines can’t cause infertility surely there’s data to support that, no? Of course! This study finds that fertility (evaluated here as fecundability) was not correlated with vaccination history, but in fact in groups at heightened risk of HPV infection, HPV vaccination was associated with superior fertility (though the effect is marginally significant). Similarly, this study finds no correlation between infertility and HPV vaccine status.
It’s worth noting additionally that the anti-vaccine lobby makes this claim with literally every vaccine and has never been correct.
Did Japan stop HPV vaccinations?
Not exactly. This is a bit complex. Professor Heidi Larson gives the context well:
In May, 2013, a cluster of adverse events suspected to be linked to HPV vaccination were reported in the Japanese media, prompting the Japanese Government to suspend proactive recommendations for the vaccine in June, 2013. Despite no evidence of a link with HPV vaccination being found in the local investigation, and calls by WHO and the global scientific community to resume active recommendation of the cancer-preventing vaccine, the proactive recommendation of HPV vaccination remains suspended in Japan.
The absence of the proactive recommendation is NOT the same as Japan stopping HPV vaccination. HPV vaccines are STILL offered in Japan, but they are not promoted to the extent that other common vaccines are. Professor Larson adds:
In 2017, the Japan Expert Council on Promotion of Vaccination—a body of 17 academic societies from a broad range of fields, including infectious disease, paediatrics, obstetrics and gynaecology, respiratory illness, travel health, and vaccinology—published a statement recommending renewed proactive support for the widespread use of the HPV vaccine.1 This important public statement was barely mentioned in the local media and had little impact on political processes.
This refusal is not without harm.
The HPV vaccine crisis to date is estimated to result in around 5000 deaths from cervical cancer in Japan [per year]. Many of these deaths could still be prevented if vaccination coverage with extended catch-up can be rapidly restored.
What about all those adverse events?
Ah yes, that old chestnut. HPV vaccines have been accused of basically every adverse event you can think of, but the occurrence of something bad after HPV vaccination does not mean that the HPV vaccine is responsible. The Institute of Medicine (IOM; now the National Academy of Medicine or NAM) has evaluated many of these adverse events in relation to the HPV vaccine to make judgments about whether or not they are related. Here’s the upshot from them:
Adverse Effects of Vaccines: Evidence and Causality (2012) Chapter: 9 Human Papillomavirus Vaccine. Nap.edu. [accessed 2021a Feb 28]. https://www.nap.edu/read/13164/chapter/11#520. Causality judgments from the IOM
For the majority of these, the causality conclusion is “inadequate” in large part because these events occur too rarely after HPV vaccination to reliably be able to make a judgment that they are caused by HPV vaccination- but that should actually be comforting. Think about it: the really bad things happen so rarely that we can’t even say they’re caused by the vaccine with certainty. That speaks to an incredible safety record on the part of the vaccine.
Certainly we know that any vaccine can very rarely cause anaphylaxis, and so the evidence favors acceptance there. There have been extensive evaluations of the safety of the HPV vaccine. In general, we know that the HPV vaccines can cause syncope, or fainting as it’s commonly called. But this isn’t actually from the HPV vaccine itself- it’s thought to be related to the anxiety of getting an injection or possibly from the pain. The age group receiving the HPV vaccine is more prone to fainting, which isn’t harmful beyond the risk of injury from the act of fainting itself, and that’s readily managed by getting the vaccine while sitting and a short waiting period. Unfortunately, this age group is also prone to psychogenic adverse reactions to vaccination, which can be real and frightening but are not the fault of a vaccine- at least in the sense of the vaccine specifically causing a reaction via some biological mechanism.
Regarding autoimmune diseases, the verdict is clear: there is no evidence to support that HPV vaccines cause autoimmune disease (and also this study), or that their receipt is associated with an increased risk of autoimmune disease. It has been looked at. Yes- I know that there are very frightening anecdotes about what happens to people after HPV vaccines. These haven’t been dismissed. These have been investigated for epidemiological links to the vaccine by independent parties who have no affiliation to the pharmaceutical companies and no link has been found. At most, these frightening tales represent extremely rare occurrence, but it’s honestly more probable from my vantage point that these events are unrelated.
References
Adverse Effects of Vaccines: Evidence and Causality (2012) Chapter: 9 Human Papillomavirus Vaccine. Nap.edu. [accessed 2021a Feb 28]. https://www.nap.edu/read/13164/chapter/11#520.
Bast RC, Hait WN, Kufe DW, Weichselbaum RR, Holland JF, Croce CM, Piccart‐Gebart M, Wang H, Hong WK, Pollock RE, editors. 2016. Holland‐Frei Cancer Medicine. Wiley.
Bednarczyk RA. 2019. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother. 15(7–8):1628–1638.
Bennett JE, Dolin R, Mandell GL, Blaser MJ, Douglas RG, (Amsterdam). E. 2020. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 9th ed. Elsevier.
Bogaards JA, Wallinga J, Brakenhoff RH, Meijer CJLM, Berkhof J. 2015. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ. 350(may12 7):h2016.
Brouwer AF, Delinger RL, Eisenberg MC, Campredon LP, Walline HM, Carey TE, Meza R. 2019. HPV vaccination has not increased sexual activity or accelerated sexual debut in a college-aged cohort of men and women. BMC Public Health. 19(1):821.
Celentano DD, Szklo M, Gordis L. 2018. Gordis Epidemiology. 6th ed. Elsevier.
Cherry J, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. 2018. Feigin and cherry’s textbook of pediatric infectious diseases: 2-Volume set. 8th ed. Philadelphia, PA: Elsevier - Health Sciences Division.
Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. 2011. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 29(46):8443–8450.
Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. 2019. Updated medical care cost estimates for HPV-associated cancers: implications for cost-effectiveness analyses of HPV vaccination in the United States. Hum Vaccin Immunother. 15(7–8):1942–1948.
Clinical Review of Biologics License Application Supplement for Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant (Gardasil®) to extend indication for prevention of vaginal and vulvar cancers related to HPV types 16 and 18. Archive-it.org. [accessed 2021b Feb 27]. https://wayback.archive-it.org/7993/20170404210128/https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111274.pdf.
DeLong G. 2018. A lowered probability of pregnancy in females in the USA aged 25-29 who received a human papillomavirus vaccine injection. J Toxicol Environ Health Part A. 81(14):661–674.
eliesbik. 2019 Aug 11. Concerns about a paper on HPV vaccination and pregnancy rates. Scienceintegritydigest.com. [accessed 2021 Feb 28]. https://scienceintegritydigest.com/2019/08/11/concerns-about-a-paper-on-hpv-vaccination-and-pregnancy-rates/.
Fainting after Vaccination. 2020 Aug 25. Cdc.gov. [accessed 2021 Feb 28]. https://www.cdc.gov/vaccinesafety/concerns/fainting.html.
Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, Chatterjee A, Iversen O-E, Joshi A, Chu J-L, et al. 2017. 4-Valent Human Papillomavirus (4vHPV) Vaccine in Preadolescents and Adolescents After 10 Years. Pediatrics. 140(6):e20163947.
Fijak M, Meinhardt A. 2006. The testis in immune privilege. Immunol Rev. 213(1):66–81.
Fortes HR, von Ranke FM, Escuissato DL, Araujo Neto CA, Zanetti G, Hochhegger B, Souza CA, Marchiori E. 2017. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med. 126:116–121.
Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley MF, et al. 2011. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 29(46):8279–8284.
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. 2011. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 364(5):401–411.
Guo F, Cofie LE, Berenson AB. 2018. Cervical cancer incidence in young U.s. females after human Papillomavirus vaccine introduction. Am J Prev Med. 55(2):197–204.
Hakenberg OW, Dräger DL, Erbersdobler A, Naumann CM, Jünemann K-P, Protzel C. 2018. The diagnosis and treatment of penile cancer. Dtsch Arztebl Int. 115(39):646–652.
How many cancers are linked with HPV each year? 2020 Sep 16. Cdc.gov. [accessed 2021 Feb 22]. https://www.cdc.gov/cancer/hpv/statistics/cases.htm.
HPV Vaccine Schedule and Dosing. 2021 Jan 26. Cdc.gov. [accessed 2021 Feb 23]. https://www.cdc.gov/hpv/hcp/schedules-recommendations.html.
Hung C-F, Ma B, Monie A, Tsen S-W, Wu T-C. 2008. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 8(4):421–439.
Iannelli V. 2017 Jul 23. Who is Diane Harper? Vaxopedia.org. [accessed 2021 Feb 27]. https://vaxopedia.org/2017/07/23/who-is-diane-harper/.
Kamidani S, Pickering LK. 2021. Human papillomavirus vaccine protects males and females against HPV-attributable cancers. AAP News. [accessed 2021 Feb 27]. https://www.aappublications.org/news/2020/04/01/mmwr040120.
Kansal NK. 2020. Immunotherapy of anogenital warts with measles, mumps, and rubella vaccine. Dermatol Ther. 33(6):e13987.
Kjaer SK, Nygård M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M, et al. 2018. A 12-year follow-up on the long-term effectiveness of the quadrivalent human Papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 66(3):339–345.
Larson HJ. 2020. Japan’s HPV vaccine crisis: act now to avert cervical cancer cases and deaths. Lancet Public Health. 5(4):e184–e185.
Li N, Wang T, Han D. 2012. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 3:152.
Marchetti RL, Gallucci-Neto J, Kurcgant D, Proença ICGF, Valiengo L da CL, Fiore LA, Pinto LF, Maranhão AGK, Oliveira MT da C, de Oliveira LH. 2020. Immunization stress-related responses presenting as psychogenic non-epileptic seizures following HPV vaccination in Rio Branco, Brazil. Vaccine. 38(43):6714–6720.
McInerney KA, Hatch EE, Wesselink AK, Mikkelsen EM, Rothman KJ, Perkins RB, Wise LA. 2017. The effect of vaccination against human Papillomavirus on fecundability. Paediatr Perinat Epidemiol. 31(6):531–536.
Meites E, Gee J, Unger E, Markowitz AL. 2020 Nov 2. Pinkbook | HPV. Cdc.gov. [accessed 2021 Feb 22]. https://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html.
Miranda S, Chaignot C, Collin C, Dray-Spira R, Weill A, Zureik M. 2017. Human papillomavirus vaccination and risk of autoimmune diseases: A large cohort study of over 2 million young girls in France. Vaccine. 35(36):4761–4768.
Moreira ED, Giuliano AR, de Hoon J, Iversen O-E, Joura EA, Restrepo J, Van Damme P, Vandermeulen C, Ellison MC, Krick A, et al. 2018. Safety profile of the 9-valent human papillomavirus vaccine: assessment in prior quadrivalent HPV vaccine recipients and in men 16 to 26 years of age. Hum Vaccin Immunother. 14(2):396–403.
Müller-Coan BG, Caetano BFR, Pagano JS, Elgui de Oliveira D. 2018. Cancer progression goes viral: The role of oncoviruses in aggressiveness of malignancies. Trends Cancer. 4(7):485–498.
Plotkin SA, Orenstein W, Offit DPA, Edwards KM. 2017. Plotkin’s Vaccines. 7th ed. Elsevier.
Portney L. 2020. Foundations of clinical research applications to evidence-based practice. 4th ed. Philadelphia, PA: F.A. Davis.
Primary ovarian insufficiency in adolescents and young women. Acog.org. [accessed 2021c Feb 27]. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2014/07/primary-ovarian-insufficiency-in-adolescents-and-young-women.
Rid A, Saxena A, Baqui AH, Bhan A, Bines J, Bouesseau M-C, Caplan A, Colgrove J, Dhai A, Gomez-Diaz R, et al. 2014. Placebo use in vaccine trials: Recommendations of a WHO expert panel. Vaccine. 32(37):4708–4712.
Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, et al. 2015. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 107(6):djv086.
Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. 2016. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2(1):16086.
Schiller J, Lowy D. 2018. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 36(32):4768–4773.
Schiller JT, Castellsagué X, Garland SM. 2012. A review of clinical trials of human Papillomavirus prophylactic vaccines. Vaccine. 30:F123–F138.
Second edition. Comprehensive cervical cancer control A guide to essential practice 2nd edition. Who.int. [accessed 2021 Feb 26]. https://apps.who.int/iris/bitstream/handle/10665/144785/9789241548953_eng.pdf?sequence=1.
Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K. 2020. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 5(4):e223–e234.
Simpson E. 2006. A historical perspective on immunological privilege. Immunol Rev. 213(1):12–22.
Smith DM, Simon JK, Baker JR Jr. 2013. Applications of nanotechnology for immunology. Nat Rev Immunol. 13(8):592–605.
Smith LM, Kaufman JS, Strumpf EC, Lévesque LE. 2015. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 187(2):E74–E81.
The use of intralesional measles-mumps-rubella vaccine in the treatment of recalcitrant viral warts. 2018. J Am Acad Dermatol. 79(3):AB294.
Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. Cdc.gov. [accessed 2021 Feb 26]. https://www.cdc.gov/mmwr/pdf/wk/mm6411.pdf#page=12.
Zhuang R-Y, Xu H-G. 2020. Head and neck cancer. N Engl J Med. 382(20):e57.