Vitamin K at Birth: Everything You Ever Wanted To Know
This is primarily written for people advocating for the use of vitamin K rather than those who many be on-the-fence about the matter, and is thus more technical by design. However, all are welcome to read it. If you are uninterested in the technical details, you can skip the “Biology of Vitamin K” (marked with a “I” before the start) sections as after that I deal with specific concerns relating to the use of Vitamin K.
Of all medical interventions, vitamin K at birth is inarguably among the most important. Yet, some individuals are refusing for several reasons:
The erroneous perception that vitamin K is a vaccine
The concern that there are toxic ingredients present in the shot
Concerns that the dose of Vitamin K is too high
Preference for “natural” sources of vitamin K
Unnecessary/lack of benefit
Pain
Religion and Personal Belief
Those refusing Vitamin K at birth were more likely to:
refuse erythromycin eye ointment at birth (given to prevent ophthalmia neonatorum, a condition which can cause permanent blindness, caused by infection of the newborn’s eyes with Neisseria gonorrheae which can be carried asymptomatically by the mother and transferred to the baby during the birthing process; the antibiotic ointment can also prevent neonatal conjunctivitis from other bacteria)
Refuse hepatitis B vaccines at birth (hepatitis B is especially devastating if contracted in infancy e.g. perinatally, having a >90% chance of progressing to a chronic form that results in hepatocellular carcinoma and liver cirrhosis requiring liver transplant to treat; the virus can survive (maintain replication competence) outside the body for over a week (some estimates say at least 1 month) and over half of people who have the disease are unaware of how they got it. Around the world, it is estimated that >90% of individuals with hepatitis B are unaware they have it. Additionally, once contracted, if not cleared by the immune system, no curative intervention currently exists, though there are treatments).
Be non-Hispanic White people
Be higher income
Exclusively breastfeed
Deliver vaginally (as opposed to C-section)
And anecdotally, I’ve seen it now circulating (pun not intended) that vitamin K thickens the blood. I believe the term typically used is “sludge.” Some parents have also argued it isn’t necessary due to cord blood, which is erroneous as well.
The refusal of vitamin K is deeply unsettling because as you will see in a moment, none of these arguments hold water. Let’s see what the science says.
Firstly, it’s not a vaccine.
It doesn’t generate a correlate of protection against any infectious disease, it doesn’t act through the immune system (although I guess that depends on how you feel about platelets as there is a collaboration between them and the clotting factors that so need vitamin K), and it’s not an antigen found on or in some pathogen. There is no meaningful definition of the term “vaccine” where one could make the argument that perinatal vitamin K is one.
Martin, R., Fanaroff, A. and Walsh, M., 2017. Fanaroff And Martin's Neonatal-Perinatal Medicine. 5th ed. Elsevier. Figure 33-1
It’s… a… vitamin.
And, it’s an incredibly important one. All babies are born with vitamin K insufficiency, and that can be catastrophic if not quickly dealt with, and a single intramuscular vitamin K injection is the absolute best way to do that.
I: The Biology of Vitamin K
Vitamin K was discovered by the Danish biochemistry Henrik Dam in the 1930s, who named it K for “Koagulations-Vitamin,” as it’s absolutely essential for blood clotting, which he discovered because chickens fed a diet lacking sterols and fats would have subcutaneous and intramuscular hemorrhages- because they didn’t clot properly. There is no singular vitamin K molecule, though. All of the following are considered forms of vitamin K (the various vitamers are shown at your immediate left).
From Robbins, S., Cotran, R., Kumar, V., Abbas, A. and Aster, J., 2020. Pathologic Basis Of Disease. 10th ed. Philadelphia, PA: Saunders Elsevier. Figure 4.4
In more formal terms, Vitamin K denotes a class of 2-methyl-1,4-naphthoquinone derivatives (which is represented by A in the figure above, also known as vitamin K3 or menadione; infants in the US receive vitamin K1, or phylloquinone, represented by B). Vitamin K is a coenzyme- an organic (meaning made primarily of carbon) chemical that helps enzymes to function. In general, Vitamin Ks are classified into vitamin K1 (phylloquinones, which are obtainable through leafy green vegetables in the diet and are the form of vitamin K given at birth), vitamin K2 (menaquinones, produced by bacteria in the gut which infants lack, and egg yolks, chicken, beef, vegetables, and fermented products, such as natto), and vitamin K3 (menadiones; these are synthetic forms of vitamin K which are no longer marketed because of toxicity concerns but had the notable advantage of being water-soluble and thus easier to administer). Humans lack the genes required for the synthesis of Vitamin K, hence it is a true vitamin like vitamin C and unlike vitamin D(s). More precisely, vitamin Ks (except for K3) are fat-soluble vitamins like vitamins A, D (not a vitamin), and E.
Ia: Coagulation, Hemostasis, Thrombosis
I think it is worth examining in detail what Vitamin K does because it really helps to explain the extent to which the previously cited concerns in the beginning of the post are detached from reality. First though, a bit of terminology:
Coagulation: the proper term for blood clotting; literally to convert to a semi-solid state
Thrombus: a blood clot that is inside a blood vessel
Hemostasis: physiological coagulation in response to vascular injury
Thrombosis: a pathological phenomenon in which a clot moves inappropriately from the site of vascular injury, potentially causing fatal complications
Embolism: obstruction of a vessel by material of some kind, usually a blood clot, but potentially fat or air.
Thrombophilia: an inappropriate inclination towards coagulation
Hemophilia: an inability to clot sufficiently, usually for a genetic reason e.g. inability to produce a functional clotting factor
The ability to clot our blood is actually extraordinarily profound and important. Consider for instance a complete inability to clot blood, a severe hemophilia for instance. Even the slightest vascular injury would have the potential to be fatal (setting aside the risk of the open wound for infection) for the potential to bleed to death (exsanguination).
On the other hand, a great deal of our pharmacopoeia is dedicated to stopping the clotting process. In those with atherosclerotic heart disease, plaques form in the arteries which obstruct blood flow. If the plaques rupture, they release material that induces clotting, and can potentially cut off the vascular supply to a tissue completely. This is the basis for a heart attack (acute myocardial infarction), as well as (ischemic) stroke (cerebrovascular accident). Sometimes clots can form from stasis of the blood (left to its own devices, blood will tend to clot- just look at a corpse) which can result in deep vein thrombosis, the formation of clots in the veins (typically the legs), which can then travel to, for instance, the lungs, causing a pulmonary embolism which can be fatal. These are known as thromboembolic events.
To fully appreciate the importance of vitamin K, one has to understand the nature of blood clotting first. Blood clotting occurs via a cascade of reactions mediated by so-called serine proteases (proteins that break down other proteins which make use of the amino acid serine to function), which are present in the blood as zymogens- inactive forms that lack biological activity and have to be converted into an active form by way of another enzyme. Blood clotting is a complex and highly regulated process that depends on platelets and a network of serine proteases (enzymes that break down proteins and require the amino acid serine in their active site to function). Let’s consider what happens when a vascular injury occurs.
As above, there is a series of concerted steps that occur in response to a vascular injury. Firstly, neurons initiate a reflex to constrict the blood vessel at the site of injury, helping to reduce blood loss. Under the endothelium of the blood vessels (the part in immediate contact with the blood) are proteins called von Willebrand factor which are bound by platelets via Gp1b/1a (glycoprotein 1b/1a), as well as collagen in the extracellular matrix. Eventually, enough of them aggregate at the site of injury to form a platelet plug, which is known as primary hemostasis. As this occurs platelets undergo a dramatic change in shape from a discoid form to a flattened one with projections and release the contents of their granules (degranulation) which include things like thromboxane A2 (a vasoconstrictor and activator of platelets), serotonin (also a vasoconstrictor), and these induce the release of endothelin-1 (the most potent vasoconstrictor known). As this happens, platelet GpIIb/IIIa (aka integrin αIIbβ3) binds fibrinogen as exposed tissue factor cleaves thrombin. Thrombin then cleaves fibrinogen into the active fibrin, which forms a mesh over the the platelets at the plug and recruits more platelets in a self-reinforcing process which is known as secondary hemostasis. To terminate the positive feedback loop (which left to its own devices would result in an ever-growing thrombus that would eventually result in an embolism), the endothelial cells start to release anticoagulants like tissue plasminogen activator (tPA; this is also a drug used in strokes caused by thrombi) and thrombomodulin, which stops the coagulation cascade. Eventually, the clot is resorbed as new extracellular matrix is laid down and the endothelial cells divide over the injury.
It’s more complicated than this but that’s the idea. Let’s consider the coagulation cascade in more detail. The figure below it presents a summary of how it all works.
From Boron, W. and Boulpaep, E., 2017. Medical Physiology. 3rd ed. Elsevier. Figure 18-12
The basic concept of the coagulation cascade is to serially amplify the steps following it, eventually forming a mesh of fibrin (factor 1a) and platelets to cover the injury so the damage can be repaired. Clotting can be induced intrinsically via contact with negatively charged surfaces (e.g. collagen in the extracellular matrix), or extrinsically via some kind of injury to the vasculature as discussed above. Endothelial cells ordinarily express multiple anticoagulant factors to prevent spontaneous clotting despite the presence of negatively charged surfaces (which repels platelets to prevent them from adhering). In a test tube however, the intrinsic pathway can be activated because of the negative charge of the glass. They are worth summarizing:
Intrinsic Pathway
Factor XII comes into contact with a negatively charged surface becoming factor XIIa (activated factor 12). This may be assisted by high-molecular weight kininogen (HMWK) to a limited extent.
Factor XIIa converts prekallikrein to kallikrein which accelerates formation of factor XIIa from factor XII.
Factor XIIa cleaves factor XI to form factor XIa which also binds HMWK.
Factor XIa cleaves factor IX to make factor IXa.
Factor IXa can cleave prothrombin (factor II) to make thrombin (factor IIa) which as described above leads to cleavage of fibrinogen to fibrin.
Factors IXa, VIIIa, IV (calcium ions), and phospholipids form a complex called tenase which cleaves factor X to make factor Xa,
Common Pathway
Lichtman, M. and Williams, W., 2017. Williams Hematology. 9th ed. New York: McGraw-Hill Medical. Figure 114-1 on the balance between coagulation and anticoagulation. Thrombin represents a link between both coagulation and anticoagulation.
Extrinsic Pathway
Injury to the vasculature exposes tissue factor (factor III) which binds factor VII to form factor VIIa.
A complex forms from factors III, VIIa, and IV similar to tenase which can convert factor X to factor Xa.
Common Pathway
Common Pathway
Factor Xa cleaves factor V to factor Va.
Factors Xa, Va, and IV form a complex called prothrombinase, which cleaves prothrombin (factor II) into thrombin (factor IIa).
Thrombin cleaves fibrinogen (factor I) to fibrin (factor Ia) and factor VIII to factor VIIIa.
Factor VIIIa facilitates conversion of factor Ia into a mesh called stable fibrin.
Thrombin catalyzes formation of more thrombin.
Thrombin activates platelets via PAR-1 (protease activated receptor-1) and PAR-4 (protease activated receptor-4) which enhance the intrinsic pathway of coagulation.
In the body, coagulation depends mostly on the extrinsic pathway.
Wiener, C., Brown, C., Houston, B., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J. and Loscalzo, J., 2020. Harrison's Principles Of Internal Medicine. 20th ed. Figure 61-3 which demonstrates the functional form of activated protein C.
Proteins C and S are also dependent on vitamin K. These are anticoagulant proteins which prevent thrombus formation. Protein C (not to be confused with C-reactive protein (CRP), though, annoyingly, protein C does also have complex, multifaceted functions in inflammation and immunity) is a proenzyme that requires vitamin K for its synthesis and protein S for its function.
Protein C can be activated in any of several ways. Firstly, via thrombin (thrombin exists in a “slow” and “fast” form which depend on the absence and presence of a sodium ion respectively, such that a drop in sodium levels promote thrombin activation of protein C, which is relevant in Virchow’s triad, or the triad of death wherein bleeding produces coagulation derangements). However, most protein C is activated via the endothelial protein C receptor (EPCR). EPCR recruits protein C in the absence of negatively charged phospholipids. As this occurs, thrombomodulin on the surface of the endothelium recruits thrombin with high affinity and causes it to cleave protein C into its active form. A ternary complex can form called protein Case from thrombin, thrombomodulin, and protein C as above which generates activated protein C. The function of protein C requires it to bind protein S, which also requires vitamin K for synthesis. When activated, protein C acts as an anticoagulant by cleaving factor Va and VIIIa into inactive forms, which terminate their ability to convert prothrombin into thrombin, and therefore cannot lead to stable fibrin formation downstream, which requires binding of protein S to activated protein C. Of note, the mutation Factor V Leiden is a common thrombophilia that occurs because Factor V cannot be cleaved by protein C. Alternatively, excess protein C results in bleeding. Protein C also binds the major inhibitor of tPA and in this way, de-inhibits anticoagulation in addition to halting coagulation, resulting in a net anticoagulative effect.
There is also a protein Z which depends on vitamin K and has an anticoagulative function but its clinical importance tends to be more limited than that of proteins C and S. It is not a protease but can directly inhibit factor X.
Ib: Vitamin K Chemical Biology and Biochemistry
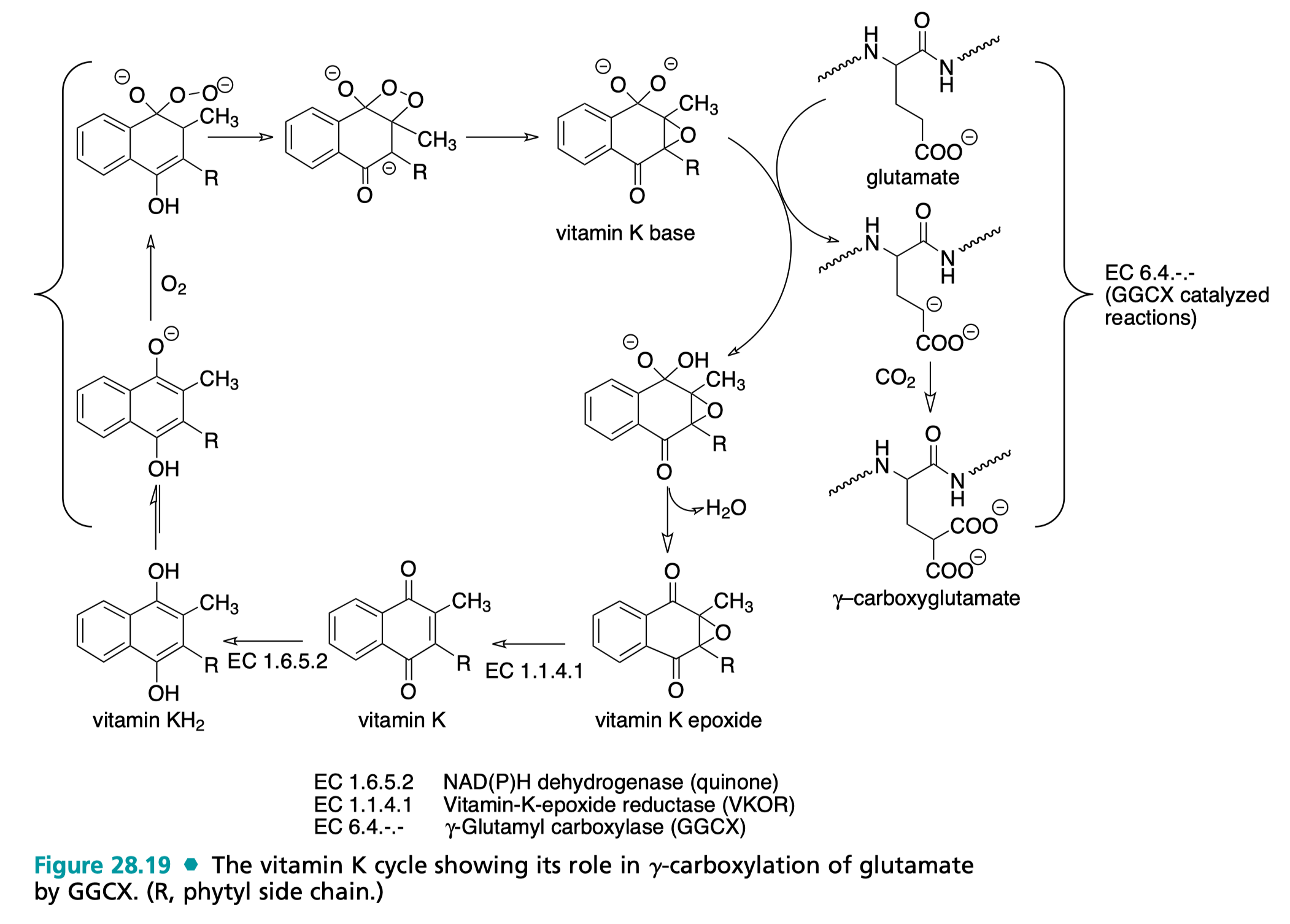
Top: The vitamin K cycle as per Wilson C, Gisvold O, Beale J. Wilson and Gisvold's Textbook of organic medicinal and pharmaceutical chemistry. 12th ed. Philadelphia, Pa.: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. Figure 28.19. See text below for details of vitamin K cycle.
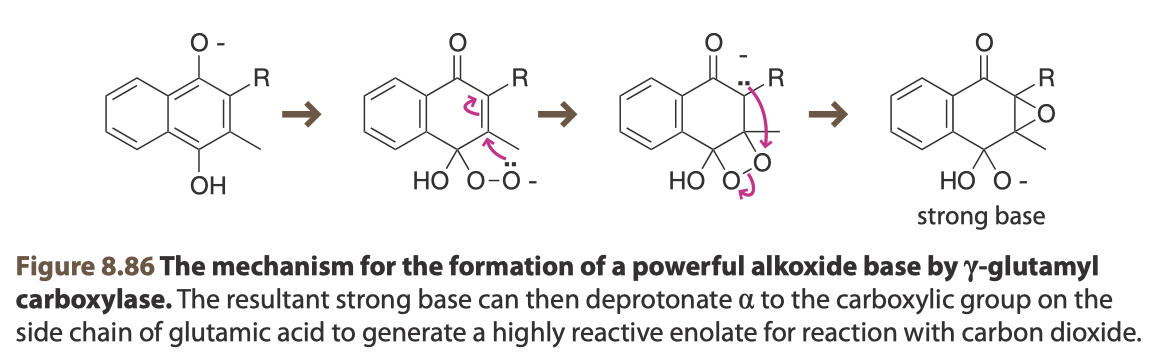
Bottom: The mechanism by which vitamin K ends up in its chemically active form (vitamin K oxide). From Van Vranken, D. and Weiss, G., 2013. Introduction To Bioorganic Chemistry And Chemical Biology. 1st ed. Garland Science, Figures 8.86
Hoffman, R., Benz, E., Silberstein, L., Heslop, H., Weitz, J., Anastasi, J., Salama, M. and Abutalib, S., 2017. Hematology Basic Principles And Practice. 7th ed. Philadelphia, PA: Elsevier. Figure 126.4 which demonstrates the Protein Case (pronounced “C”-”ase”) complex composed of thrombomodulin, protein C, and factor IIa (thrombin) which produces activated protein C, the major effector protein of anticoagulation which also depends on vitamin K.
At the biochemical level, vitamin K compounds catalyze carboxylations (reactions that add a carboxylate group to a given position). Carboxylations of the γ-carbon of glutamate’s side chain creates a moiety that can effectively bind calcium ions (the negative charges on the carboxylate group chelate the positively charged calcium ions). In the enzyme prothrombin, this leads to a conformational change that that creates a binding pocket for phosphatidylserine (PS), a lipid that is normally present on the inside of cells and indicates damage to the cells. γ-carboxyglutamate is present on Factors II, VII, IX, and X which as above are involved in coagulation, and proteins C, S, and Z which are involved in anticoagulation. These are all represented in Figure 126.2 and share the Gla domain located at the N-termini as shown below. There are additionally a few more proteins bearing Gla domains that are vitamin K-dependent but do not function in coagulation (though notably, osteocalcin and matrix Gla protein are important in normal bone health, and thus warfarin, a mimic of vitamin K which lacks its catalytic potential, can also have adverse effects on bone health by promoting mineral loss and bone malformation; vitamin K vitamers can also affect the transcription of mRNAs in osteoblasts to regulate bone-specific genes in addition to their catalytic role).
Vitamin K functions via catalytic cycle which can be thought of as beginning with its quinone form and then undergoing a series of enzyme-mediated redox reactions that culminated in the formation of γ-carboxyglutamate on target proteins. Initially, the vitamin K vitamer undergoes a hydrogenation via NAD(P)H dehydrogenase (the true identity of the enzyme, or enzymes, responsible for this conversion are not known but is also commonly referred to as Vitamin K reductase) to convert to a hydroquinone (KH2), which can spontaneously deprotonate (pKa of the resultant base is ~9, which is insufficient to deprotonate the γ-carbon, requiring a pKa of about 26-28). Following this, an oxygen diradical (ubiquitous) can be conjugated and spontaneously forms a highly strained 4-member ring via a Michael reaction as shown in Figure 8.86 that results in a carbanion. The carbanion then bonds with the oxygen and breaks the very weak peroxy bond to form an epoxide and strong base (which can deprotonate the γ-carbon). This form of vitamin K is now primed for its principal catalytic activity. The γ-carbon of the proprotein glutamate contains labile protons which can be removed to produce another carbanion. γ-carboxyglutamyl carboxylase (GGCX) can then catalyze a conjugation at the γ position of the side chain of carbon dioxide (also fairly ubiquitous) to generate a new carboxy group, producing γ-carboxyglutamate. Finally, a dehydration of the vitamin K vitamer occurs (as geminal diols are unstable) and vitamin K epoxide reductase (VKOR) converts vitamin K back into the quinone form, where it can undergo another cycle of carboxylation. This reaction does not require the use of ATP, instead being driven by the oxidation of KH2 (reducing power is also a critical form of currency in the cell, primarily generated via the pentose phosphate pathway).
All of the glutamates are carboxylated for the clotting factor to be functional. The chelation of the calcium is what allows the clotting factor to bind negatively charged substrates (which is required for secondary hemostasis, as this is how they bind the phospholipids on the platelet plasma membrane). Therefore, insofar as at a minimum, thrombin (factor IIa) is required for the formation of fibrin, vitamin K is indispensable for hemostasis and normal response to injury.
Ic: Metabolism of Vitamin K
Absorption of vitamin K occurs in the gut but the details of this process are not completely characterized. Being a fat-soluble vitamin, it is packaged with other fatty substances into chylomicrons which are absorbed through the small intestines and then drain into the liver via the hepatic portal vein, and can be recirculated enterohepatically, resulting in an overall bioavailability of 40-80%, depending on the extent of cycling and vehicle. Initial absorption of vitamin K1 is known to occur mostly in the proximal intestines and kinetics indicate that this occurs via a saturable, energy-requiring transport process. Three proteins are implicated in this process:
class B scavenger receptors SRB1 and CD36: broad specificity and known to be used for cholesterol and Vitamin E; in mice, overexpression does appear to enhance uptake
Niemann-Pick type C1-like 1: NPC1L1, known to be a transporter for cholesterol and α- tocopherol; evidence limited to cell lines
It can also be packaged into low-density lipoproteins after the chylomicrons are taken up by hepatocytes and there circulate to the bone marrow. Though there is vitamin K stored in the liver, unlike other fat-soluble vitamins, there is very rapid turnover of vitamin K as it undergoes its epoxide cycle (summarized above) to produce Gla domains in the appropriate proteins. The liver is also where most vitamin K ends up when administered parenterally. With the exception of the menadiones, vitamin K is a fat-soluble vitamin, however, and thus also has been noted to accumulate within the cell membranes of the adrenal glands, bone marrow, lungs, and kidneys. Because vitamin K turnover in the liver is so rapid, effective storage of vitamin K does not occur, and a continuous supply of vitamin K is necessary. Catabolism of the vitamers occurs via ω- and β-oxidation pathways. More detailed discussions of vitamin K metabolism can be found here, here, and here.
From Hoffman, R., Benz, E., Silberstein, L., Heslop, H., Weitz, J., Anastasi, J., Salama, M. and Abutalib, S., 2017. Hematology Basic Principles And Practice. 7th ed. Philadelphia, PA: Elsevier Figure 126.2
II: Vitamin K Deficiency Bleeding of Early Infancy (formerly known as Hemorrhagic Disease of the Newborn)
Vitamin K deficiency occurs almost exclusively in infants. It is very unusual for an adult to have a vitamin K deficiency, but it can happen in a few ways. Antibiotics can result in the death of commensal species which make vitamin K, and thus create a state of deficiency. Additionally, certain malabsorption disorders exist which can prevent absorption of vitamin K to the liver (where it is used to help synthesize functional proenzyme clotting factors) which could cause deficiency. It has also been noted with renal failure for reasons not fully understood, and in liver cirrhosis (as the liver is a principal compartment for storage of vitamin K). The outcomes of this deficiency are worth discussing.
I hope I have at this point satisficed any queries about whether or not vitamin K is in fact necessary for normal blood clotting. By virtue of the fact that vitamin K is required for synthesis of thrombin, let alone the other critical factors of the coagulation cascade, it should be more than sufficient to explain that a deficiency of vitamin K- depending on the extent- can result in profound and potentially catastrophic bleeding.
Vitamin K deficiency bleeding (VKDB) was previously called “hemorrhagic disease of the newborn.” VKDB has 3 basic forms:
Early VKDB occurs within 24 hours of birth. It is more likely to occur in mothers who have used certain drugs e.g. anticonvulsants (phenytoin, barbiturates, carbamazepine), some antituberculosis medications (isoniazid, rifampin), some antibiotics (cephalosporins) and warfarin (which as you will note above, profoundly interferes with the action of vitamin K). Pregnant mothers who are taking such medications are recommended to supplement vitamin K during the last 2-4 weeks of pregnancy, as the birth dose of vitamin K given within 24 hours is not always sufficient to protect against early bleeding. Unfortunately, though low-risk, it is not clear that prenatal supplementation with vitamin K close to delivery actually helps to prevent vitamin K (though case reports from the Japanese Society of Obstetrical, Gynecological, and Neonatal Hematology from 2017 examining cases from 2007 to 2016 noted that in none of the 20 cases did the mothers receive supplemental vitamin K). Early VKDB typically involves bleeding in the skull, including over the brain, but also within the chest cavity and in the abdomen as well.
Classic VKDB most commonly occurs after the first day but can occur at any point after the first day until the first week and can overlap with late VKDB. Children at risk for classic VKDB may appear to be ill near birth and have delayed feeding. However, parents should not wait to see if this occurs as by this point administration of protective vitamin K may be too late. Bleeding may occur in the intestines (resulting in tarry black stool called melena), near the umbilical cord, skin (bruising), nose, and at the circumcision site. Classic occurs in part because in the absence of intervention, there is a worsening of vitamin K deficiency in the first week of life. The prognosis for classic bleeding tends to be better because it occurs in the gastrointestinal tract rather than in the brain as is common with late and early VKDB. Exclusive breastfeeding is also a risk factor for classic bleeding (see below).
Early and classic VKDB together occur in as many as 1 in 60 live births (lower bound 1 in 250), and if the mother takes medications that can predispose towards early VKDB stated above, that risk can be dramatically higher. Vitamin K deficiency bleeding is not that rare.
Late VKDB by comparison is by comparison relatively rare, occurring at a rate of about 1 in 14,000 to 1 in 25,000 infants from day 8 of life until up to 6 months. Risk factors include malabsorption conditions including cystic fibrosis, biliary atresia, chronic diarrhea, antibiotic therapy, α1-antitrypsin deficiency, and rarely, antibiotic therapy. Bleeding is typically severe and there aren’t warning bleeds. 30-60% of cases involve bleeding in the brain. Babies who are exclusively breastfed are at higher risk for late VKDB than formula-fed babies. Breastmilk contains 1-4 μg/L of vitamin K1 with almost no vitamin K2, which is not nearly enough, as a neonate requires approximately 2 μg per day, but does not consume anything close to a liter of milk (and this assumes 100% oral bioavailability of the vitamin which is not the case; see IV: Why can’t I give vitamin K orally?). Colostrum has about 2 μg/L of Vitamin K and typically has a volume of about 90 mL cumulatively (it is only produced on the first, second, and third days of life) for a total 0.18 μg of Vitamin K which is also insufficient. Formula on the other hand is enriched with vitamin K (however, intramuscular injection of vitamin K within the first day of life is still the gold standard). Similarly, there is little to no vitamin K in cord blood (<0.05 μg/L ).
Newman, P. and Shearer, M., 2008. Metabolism and cell biology of vitamin K. Thrombosis and Haemostasis, 100(10), pp.530-547. Figure 2; Presents an overview of the transport of vitamin K throughout the body.
Prognosis for VKDB is variable but generally poor. If intracranial bleeding occurs, 20-50% of affected children will die and sequelae are likely. A study of 126 cases showed a fatality ratio of 26%.
III: Why are babies born Vitamin K deficient? If VKDB is so serious, how did humans survive this long?
Regrettably, all infants are born with a vitamin K deficiency. We can examine why in two basic ways.
Table 2 from Shearer, M. J. (2009). Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Reviews, 23(2), 49–59. doi:10.1016/j.blre.2008.06.001
Firstly, the proximal one, which is threefold: vitamin K does not effectively cross the placenta, infants are not yet colonized by the gut microbes that produce vitamin K (and furthermore their gut is somewhat immature and can have difficulties absorbing it enterically, particularly if they have cholestasis), and humans lack the genes required to synthesize vitamin K de novo. By virtue of these facts alone, regardless of how much phylloquinone is ingested, how much menaquinone is made by the pregnant mother’s gut microbiota, or even how much vitamin K a pregnant woman theoretically receives, there is no significant effect on the ability of the newborn to clot (even though there seems to be a rise in the level of vitamin K in cord blood, there is no change in the levels of clotting factors; this goes back to the point about how evidence regarding whether or not vitamin K supplementation of the mother to prevent early VKDB is equivocal regarding efficacy). Vitamin K has a very steep gradient as far as its transport across the placenta of about 40:1 (meaning that for every 40 molecules of vitamin K, 1 may cross the placenta). Additionally, vitamin K is incorporated and turned over in the liver very rapidly (relatively speaking) so at any given moment its concentration in the blood is low.
Per H, Arslan D, Gumus H, Coşkun A, Kumandas S. Intracranial Hemorrhages and Late Hemorrhagic Disease Associated Cholestatic Liver Disease. Pediatric Research. 2010;68:396-396.
As for the more profound evolutionary “why,” that’s harder and not well understood. Vincent Racaniello famously states that we cannot answer “why” questions in biology, but I can offer a bit of good-natured speculation. There is likely good reason for mothers to hold on to their Vitamin K, when one considers how bloody parturition is. It is often argued that humans are born premature, in comparison to other mammals: we cannot walk, talk, etc. We are born lacking many fundamental features required for survival, and thus we rely heavily on our parents. A mother that has bled to death will have trouble providing her infant the nourishment needed for them to thrive. Though of course, the question of why we are born so premature is another evolutionary can of worms but I would guess it has to do with the mother-infant pair bond primates have evolved which allows for it.
Ultimately though, no one can say with certainty why babies are born deficient in vitamin K. What we can say with certainty is that it’s a huge problem that needs rapid correction within 24 hours of birth to prevent potentially catastrophic outcomes.
IV: Why can’t I give vitamin K orally?
There’s a geographic element here as there are parts of the world which do use oral vitamin K to prevent VKDB. However, as far as preventing vitamin K deficiency bleeding, the intramuscular injection is the gold standard. There is a stringent dosing schedule that has to be adhered to for the first few weeks of the infant’s life if the oral version is used and adding another responsibility to sleep-deprived new parents when an alternative exists is simply unwise. Oral bioavailability of vitamin K is variable as the gut of the infant is still immature, but tends to be around 80% whereas it is ~100% by the intramuscular route (eventually). Additionally, absorption is more effective via the intramuscular route; infants with certain gallbladder problems (cholestasis) are unable to effectively absorb oral vitamin K.
As summarized here:
Since the return to the recommendation for intramuscular prophylaxis in 1994, there have been four cases of VKDB after incomplete oral dosing. Lack of compliance with three oral doses is recognised as a potential problem in Australia 38 and elsewhere, 39 but has been dismissed by others. 40 Intravenous vitamin K prophylaxis does not provide the lasting protection achieved with intramuscular vitamin K 19 and has never been recommended in Australia, although it was given alone to one infant in 2004, and in combination with other modes of administration to three children between 1993 and 1994.
And as described here:
The associated incidence of late VKDB has, however, repeatedly been observed to be higher when compared to a single 1 mg IM dose at birth (23,68,76,77).
In the US, there is no standardized vitamin K oral formulation and it would be a supplement, which does not face the same degree of regulation (honestly it’s very nominal) as do pharmaceutical drugs. Thus it is theoretically possible for a vitamin K oral supplement to entirely lack vitamin K.
V: Toxicity
Hypervitaminosis K is extremely rare. It generally does not occur unless one consumes >1000 times the daily requirement of vitamin K. Though it is fat-soluble, there is extremely rapid turnover of vitamin K in the liver and thus it is hard for the vitamin to build up. It also essentially does not occur with vitamin K1 or K2, and vitamin K3 is no longer in use. In the case of vitamin K3, the vitamin seems to enhance delivery of oxygen to the liver, which enhances lipid peroxidation and causes cell death and tissue damage. Toxicity is in fact so rare that the Food and Nutrition Board is not able to establish a toxic level of vitamin K.
Suffice it to say, the dose of Vitamin K needed to achieve this is well beyond the approximately 0.5 to 1 mg Vitamin K given to term infants IM within 24 hours of birth.
There is one case worth discussing regarding toxicity: glucose-6-phosphate dehydrogenase (G6PD) deficiency. This is a relatively common genetic variant in which an enzyme, G6PD, does not function as effectively as it should which downstream impedes the ability to make glutathione and makes cells more vulnerable to oxidative stress. The salient point however is here:
Taking into account the scarcity of reports and widespread use of vitamin K after birth among infants, it is likely that vitamin K can be administered safely to G6PD deficient individuals.
To add a bit more detail, at birth, a term infant receives 1 mg Vitamin K by intramuscular injection. One would note however that recommended daily allowance of vitamin K for a newborn infant is 2 μg. However, the IM injection forms a depot that slowly dissolves out vitamin K, where it reaches a peak concentration and then drops as shown here:
SOEDIRMAN J, DE BRUIJN E, MAES R, HANCK A, GRÜTER J. Pharmacokinetics and tolerance of intravenous and intramuscular phylloquinone(vitamin K1) mixed micelles formulation. British Journal of Clinical Pharmacology. 1996;41(6):517-523. Figure 2 a-f
The area under the curve gives the total quantity of vitamin K which will eventually equal 1000 μg (1 mg).
VI: Vitamin K and Cancer
There is actually some fascinating biochemistry involved here. There were some small studies which initially seemed to suggest a link between vitamin K and some forms of leukemia. First the key point: substantially larger studies have failed to reproduce this effect, and given that the initial studies finding the signal of concern were highly underpowered, suggests that the initial signal was artefact and next to the risk of VKDB is wholly inadequate to recommend against universal supplementation. But there’s actually some basis to think that vitamin K may even be protective against cancer. GAS6 is a vitamin K-dependent protein whose expression increases by a factor of 30 during the G0 phase of the cell cycle (not a true phase but instead referring to a long-term quiescent state cells can enter without dividing i.e. senescence). There is some literature which notes that reduced expression of this protein is associated with some cancers, and furthermore was noted to even possible be protective against hepatocellular carcinoma. This should not be taken as licensure to megadose vitamin K as cancer prevention or any other reason. As described here:
… although VK supplementation improves biochemical markers of VK status, the evidence for benefits on specific health outcomes beyond coagulation are equivocal.
VII: Does vitamin K thicken the blood?
From Boron, W. and Boulpaep, E., 2017. Medical Physiology. 3rd ed. Elsevier. Figure 18-12
No. I really hate this question because it’s a wrong question. Formally, the thickness of a fluid refers to its viscosity.
Viscosity is the relationship between the shear stress (the force needed to move one sheet of fluid faster than its neighbor) and the shear rate (the difference in the speed of different adjacent sheets of fluid). A fluid is said to be Newtonian if the relationship is linear. Blood, however, is not linear, though you will note that both water and plasma are, and such fluids are said to be non-Newtonian. In practice, the viscosity of a given sample of blood depends on:
Fibrinogen (factor I) concentration
Hematocrit (proportion of the blood made up by red blood cells, RBCs)
Vessel radius
Linear velocity of the blood sample
Temperature
The first two are worth discussing further. A higher hematocrit results in more viscous blood, as shown above with the polycythemia curve. The non-Newtonian nature of blood as a fluid arises largely from the interaction of fibrinogen with the RBCs. At physiological concentrations, fibrinogen (and the lipoproteins of LDL) are the only proteins that can exert shear stress in the blood. If fibrinogen concentrations rise drastically, RBCs cluster and their effective density increases. The level of fibrinogen tends to rise with age, and will increase from smoking. Similarly, at a hematocrit of 60% or above (normal range: for men is 40 - 54%; for women it is 36 - 48%) profound clustering of the RBCs occurs and drastically raises viscosity. The non-Newtonian properties tend to arise when you reach a hematocrit of 30%.
You will note that nowhere here is vitamin K mentioned. This is because vitamin K has nothing to do with the thickness of blood in the formal sense. Vitamin K is not used for the manufacture of factor I (fibrinogen; see above) and thus cannot affect blood viscosity.
I think that people most often refer to the fact that a sudden dose of vitamin K that comes from injection is going to cause spontaneous clot formation throughout the body which is… very incorrect. For one thing, vitamin K has to first get to the liver and be incorporated into Gla domain-bearing proteins for them to be functional. Additionally, clot formation does not simply occur from the presence clotting factors in the blood. For one thing, the proteins of the clotting cascade are not functional at baseline in the blood because they are present as proenzymes and require proteolytic activation to do anything. This does not occur spontaneously (see the clotting cascade above for a detailed explanation). It typically requires injury to expose von Willebrand factor and tissue factor (extrinsic pathway) in the body (intrinsic pathway induced coagulation is comparatively rare). In short, vitamin K is required for licensure of clotting but does not itself cause clotting or affect the viscosity of blood to any significant extent:
The literal warning from the vitamin K package insert that says it will not spontaneously induce clotting e.g. if someone is bleeding. Vitamin K is not a styptic.
VIII: But the Vitamin K is synthetic, and it’s got all those other crazy ingredients!
This is the naturalistic fallacy- the perception that something which is natural must be good and something which is unnatural is not. Formally, Vitamin K1 and K2 are both naturally occurring (from plants and bacteria respectively). I have seen some argue that the Phytonadione given to infants at birth is not the same as the natural phylloquinone. These are both vitamin K1. Phytonadione is a -dione though (it has two carbonyl groups (=O)) and phylloquinone has two alcohols (-OH) at the site where the carbonyl groups would be. Phytonadione is converted into phylloquinone in the vitamin K catalytic cycle. It is no less natural than phylloquinone. Phytonadione however is more readily primed for the formation of the vitamin K base which allows it to, in principle, generate clotting factors more rapidly (see above).
Whether or not a substance has a natural or synthetic origin has absolutely no bearing on how toxic it is, and this distinction is increasingly becoming blurry. For instance, we can force bacteria to manufacture essentially any protein, including one humans make, that we would like, even if the proteins do not naturally occur in bacteria, and we can even do this by taking advantage of the natural processes that bacteria themselves use to exchange genes. The process by which this is done is entirely natural. Bacteria have an inherent ability to produce protein which is very similar to ours (there is some difference in the structures involved compared with ours, which can be used effectively with some antibiotics) This is done in the production of insulin. Before this, it took huge amounts of pig pancreases (and naturally dead pigs) to make even tiny quantities of insulin. The absurdity of the concept is however very well illustrated with a commentary that appeared here. We can define natural as being free of human intervention, with more human intervention equating to being less natural. Consider tomato paste. Most would agree this is a reasonably natural product (we will allow that the tomato in question is non-GMO, was made without pesticides, and there was no role of selective breeding of any kind in its production). The only human intervention here was the actual processing of the tomato fruit into a paste, so one could perhaps argue that a tomato is more natural than the paste we made from it. Now suppose we add sugar to it. Many would contend this is now unnatural. But now suppose we use a process to separate out the sugar such that the composition of this tomato paste is equivalent to that of the tomato paste before the addition of the sugar. If we judge by composition alone, this tomato paste is now more natural then the predecessor paste with added sugar. Except that it has undergone MORE intervention by humans to be produced than the tomato paste with added sugar and therefore by our criteria must be less natural. As you can see, the concept has no real meaning and is completely contrived. It also has no toxicological meaning.
Regarding the specific components of the shot:
Each 0.5 mL of Phytonadione Injectable Emulsion, USP contains the following inactive ingredients: 10 mg polysorbate 80, 10.4 mg propylene glycol, 0.17 mg sodium acetate anhydrous, and 0.00002 mL glacial acetic acid. Additional glacial acetic acid or sodium acetate anhydrous may have been added to adjust pH to meet USP limits of 3.5 to 7.0. The air above the liquid in the individual containers has been displaced by flushing with nitrogen during the filling operation.
The shot contains 1 mg Vitamin K1 in a volume of 0.5 milliliters (about 10 drops of water)
10 mg polysorbate 80 (PS80): This is used as an emulsifier. Vitamin K1 is very greasy and does not dissolve well in water or water-based solutions. The addition of PS80 allows for effective dissolution.
10.4 mg propylene glycol helps to ensure that the vitamin K does not dry out by pulling water to it. It is generally recognized as safe by the FDA.
0.17 mg sodium acetate anhydrous and 0.00002 mL glacial acetic acid form a buffer (a solution that is resistant to pH changes because addition of acid or base results in a corresponding increase in the conjugate) to keep the pH within a prescribed range of 3.5 to 7.0.
Benzyl alcohol as a preservative in Bacteriostatic Sodium Chloride Injection has been associated with toxicity in newborns. Data are unavailable on the toxicity of other preservatives in this age group. There is no evidence to suggest that the small amount of benzyl alcohol contained in Vitamin K1 Injection (Phytonadione Injectable Emulsion, USP), when used as recommended, is associated with toxicity.
There is a very small amount of aluminum present in the vitamin K shot. It is not added, it is residual aluminum because virtually all containers used in the manufacturing of pharmaceuticals contain aluminum.
There is also a preservative-free version of vitamin K available upon request. However, one should bear in mind that preservatives are present to prevent the contamination of the product with bacteria, which, in a newborn, could be very dangerous.
The shot is safe. The ingredients are safe. Give your newborn the vitamin K shot.
IX: We’re not circumcising so we don’t need vitamin K.
Even though circumcision is a risk factor for classic VKDB, vitamin K is administered to all children irrespective of biological sex because all babies are born with vitamin K deficiency.
Also as a general rule, please don’t just tell random people about your children’s genitals (but do discuss that sort of thing with your child’s pediatrician).
X: But it’s not 100% effective! I could do everything right and my child could get vitamin K deficiency bleeding anyway!
This is, unfortunately, a true statement. Sometimes we can do everything right and still, the worst happens. Why?
Firstly, in cases of liver disease or certain genetic conditions (e.g. hemophilias), even vitamin K given IM may not be sufficient to prevent VKDB, unfortunately (as the liver is unable to effectively use the vitamin K to make clotting factors). In the case of early VKDB, this is usually due to the use of certain medications by the mother (most notably warfarin, but also anticonvulsants), and so some suggest that 1-2 mg of vitamin K be administered intravenously in these circumstances (to more rapidly boost production of clotting factors); if this fails to improve the prothrombin time or evidence of bleeding occurs, fresh-frozen plasma should be considered.
Recent work demonstrates that exclusively breastmilk-fed infants show biochemical evidence of vitamin K deficiency (i.e., PIVKA proteins) in their blood despite intramuscular vitamin K near birth, but this is not currently known to translate to actual clinical problems. This does reflect the relative lack of vitamin K in breastmilk, however, and raises questions about the need for additional supplementation.
However, vitamin K IM supplementation within 24 hours of birth reduces the risk of VKDB by a factor of 81. That’s not perfect, sure, and as discussed earlier, with early VKDB in particular, prevention can be a challenge, but it means that parents have the power to give their newborns an incredible degree of protection with a very simple intervention.
XI: What about the risks and Black Box warning?
An absolutely appropriate and reasonable question. The fact is that vitamin K is an essential nutrient and overdosing on it is extraordinarily difficult (see above), the risks are almost nonexistent. Being an injection, it does cause some pain and there can be some bleeding at the injection site. There is a Black Box warning on some of the package inserts but the meaning of this has been wildly misinterpreted. The presence of a black box warning does not equate to a product being dangerous. The point of the warning is to call to the attention of the prescriber the most important risks of using a particular pharmaceutical and situations where the risks of its use may exceed its benefits. All pharmaceuticals carry some level of risk- this is unavoidable. However, as for the actual adverse events in newborns that have occurred following administration of vitamin K1 (phytonadione), the results are not so dramatic. As it applies to the newborn:
There has been one case of anaphylaxis (of millions of doses) documented with the newborn vitamin K IM injection (probably not caused by the vitamin K itself). Anaphylaxis can occur from virtually anything, but is very rare with the IM injection. Almost all allergic reactions to vitamin K occur with the IV administration. IV administration of vitamin K is not performed unless there is vitamin K deficiency bleeding.
At doses higher than the recommended quantity, hyperbilirubinemia and jaundice can rarely occur.
That’s it. It’s about as benign an adverse effect profile as exists for any drug.
The Upshot:
Vitamin K is absolutely critical and should be given within 6 hours of birth to prevent vitamin K deficiency bleeding (VKDB). It has virtually no risks associated with its use and corrects a universal vitamin deficiency in neonates that can have disastrous consequences which are potentially fatal. Concerns about the injection are unfounded. Intramuscular vitamin K is the ideal method for correcting neonatal vitamin K deficiency to prevent VKDB and should be used whenever available, with oral vitamin K in circumstances when intramuscular is not possible to use.
References
Boron W, Boulpaep E. Medical Physiology. 3rd ed. Elsevier; 2017.
Hamrick H, Gable E, Freeman E, Dunn L, Zimmerman S, Rusin M, Linthavong O, Wright M, Moss L, Skinner A. Reasons for Refusal of Newborn Vitamin K Prophylaxis: Implications for Management and Education. Hospital Pediatrics. 2015;6(1):15-21.
Hoffman R, Benz E, Silberstein L, Heslop H, Weitz J, Anastasi J, Salama M, Abutalib S. Hematology Basic Principles and Practice. 7th ed. Philadelphia, PA: Elsevier; 2017.
Kapoor V, Whyte R, Vedula S. Interventions for preventing ophthalmia neonatorum. Cochrane Database of Systematic Reviews. 2016.
Lichtman M, Williams W. Williams Hematology. 9th ed. New York: McGraw-Hill Medical; 2017.
Loyal J, Taylor J, Phillipi C, Goyal N, Wood K, Seashore C, King B, Colson E, Shabanova V, Shapiro E. Factors Associated With Refusal of Intramuscular Vitamin K in Normal Newborns. Pediatrics. 2018;142(2):e20173743.
Martin R, Fanaroff A, Walsh M. Fanaroff and Martin's neonatal-perinatal medicine. 5th ed. Elsevier; 2017.
Nelson N, Easterbrook P, McMahon B. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clinics in Liver Disease. 2016;20(4):607-628.
Newman P, Shearer M. Metabolism and cell biology of vitamin K. Thrombosis and Haemostasis. 2008;100(10):530-547.
Ochoa K, Mendez M. Ophthalmia Neonatorum. Ncbi.nlm.nih.gov. 2020.
Robbins S, Cotran R, Kumar V, Abbas A, Aster J. Pathologic Basis of Disease. 10th ed. Philadelphia, PA: Saunders Elsevier; 2020.
Van Vranken D, Weiss G. Introduction to bioorganic chemistry and chemical biology. 1st ed. Garland Science; 2013 p. 376-377.
Why Infants Should Receive the Hepatitis B Vaccine at Birth | Shot of Prevention. Shot of Prevention. 2020.
Wiener C, Brown C, Houston B, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's principles of internal medicine. 20th ed.; 2020.
Wolfe C, Hamborsky J, Mclntyre L, Kroger A. Epidemiology & prevention of vaccine-preventable diseases. 13th ed.; p. 149-174.
Araki S, Shirahata A. Vitamin K Deficiency Bleeding in Infancy. Nutrients. 2020;12(3):780.
Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiological Reviews. 1982;46(3):241-280.
Colostrum is the Milk Your Baby Gets the First Time You Breastfeed. Verywell Family. 2020.
Fusaro M, Plebani M, Iervasi G, Gallieni M. Vitamin K Deficiency in Chronic Kidney Disease: Evidence Is Building Up. American Journal of Nephrology. 2016;45(1):1-3.
Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. The Lancet Gastroenterology and Hepatology. 2016;3(6):383-409.
Hepatitis B Treatment. Hep. 2020.
Lippi G, Franchini M. Vitamin K in neonates: facts and myths. Blood Transfus. 2011;9:4-9.
Miller H, Kerruish N, Broadbent R, Barker D, Wheeler B. Why do parents decline newborn intramuscular vitamin K prophylaxis?. Journal of Medical Ethics. 2016;42(10):643-648.
Office of Dietary Supplements - Vitamin K. Ods.od.nih.gov. 2020
Olson R. The Function and Metabolism of Vitamin K. Annual Review of Nutrition. 1984;4:281-337.
Pearson R. Among the Vitamin K ‘Anti-Vaxxers’. The New York Review of Books. 2020.
Sankar M, Chandrasekaran A, Kumar P, Thukral A, Agarwal R, Paul V. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. Journal of Perinatology. 2016;36(S1):S29-S35.
Shearer M. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Reviews. 2009;23(2):49-59.
Shearer M. Vitamin K metabolism and nutriture. Blood Reviews. 1992;6(2):92-104.
Viral Hepatitis in the United States: Data and Trends. HHS.gov. 2020.
1. Wilson C, Gisvold O, Beale J. Wilson and Gisvold's Textbook of organic medicinal and pharmaceutical chemistry. 12th ed. Philadelphia, Pa.: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
SOEDIRMAN J, DE BRUIJN E, MAES R, HANCK A, GRÜTER J. Pharmacokinetics and tolerance of intravenous and intramuscular phylloquinone(vitamin K1) mixed micelles formulation. British Journal of Clinical Pharmacology. 1996;41(6):517-523.
Miller H, Kerruish N, Broadbent R, Barker D, Wheeler B. Why do parents decline newborn intramuscular vitamin K prophylaxis?. Journal of Medical Ethics. 2016;42(10):643-648.
Committee on Fetus and Newborn. Controversies Concerning Vitamin K and the Newborn. PEDIATRICS. 2003;112(1):191-192.
Mihatsch W, Braegger C, Bronsky J, Campoy C, Domellöf M, Fewtrell M, Mis N, Hojsak I, Hulst J, Indrio F et al. Prevention of Vitamin K Deficiency Bleeding in Newborn Infants. Journal of Pediatric Gastroenterology & Nutrition. 2016;63(1):123-129.
Ng E, Loewy A. Guidelines for vitamin K prophylaxis in newborns. Paediatrics & Child Health. 2018;23(6):394-397.
Cornelissen M, von Kries R, Loughnan P, Schubiger G. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K. European Journal of Pediatrics. 1997;156(2):126-130.
Shahrook S, Ota E, Hanada N, Sawada K, Mori R. Vitamin K supplementation during pregnancy for improving outcomes: a systematic review and meta-analysis. Scientific Reports. 2018;8(1).
Xiao J, Melvin R, Salsbury F. Mechanistic insights into thrombin's switch between “slow” and “fast” forms. Physical Chemistry Chemical Physics. 2017;19(36):24522-24533.
Witt M, Kvist N, Jorgensen MH, et al. Prophylactic Dosing of Vitamin K to Prevent Bleeding. Pediatrics. 2016;137(5):e20154222. PEDIATRICS. 2016;138(4):e20162475-e20162475.
Per H, Arslan D, Gumus H, Coşkun A, Kumandas S. Intracranial Hemorrhages and Late Hemorrhagic Disease Associated Cholestatic Liver Disease. Pediatric Research. 2010;68:396-396.
Ibrahim A, Gray Z, Gomes A, Myers L, Behbod F, Machado H. Gas6 expression is reduced in advanced breast cancers. npj Precision Oncology. 2020;4(1).
Ekelund H, Finnstrom O, Gunnarskog J, Kallen B, Larsson Y. Administration of vitamin K to newborn infants and childhood cancer. BMJ. 1993;307(6896):89-91.
Jinghe X. Vitamin K and hepatocellular carcinoma: The basic and clinic. World Journal of Clinical Cases. 2015;3(9):757.
Booth S. Roles for Vitamin K Beyond Coagulation. Annual Review of Nutrition. 2009;29(1):89-110.
Tie J, Stafford D. Structural and functional insights into enzymes of the vitamin K cycle. Journal of Thrombosis and Haemostasis. 2016;14(2):236-247.
ZIPURSKY A. PREVENTION OF VITAMIN K DEFICIENCY BLEEDING IN NEWBORNS. British Journal of Haematology. 1999;104(3):430-437.
Zurynski Y, Grover C, Jalaludin B, Elliott E. Vitamin K deficiency bleeding in Australian infants 1993–2017: an Australian Paediatric Surveillance Unit study. Archives of Disease in Childhood. 2019;105(5):433-438.
Britt R, Brown J. Characterizing the Severe Reactions of Parenteral Vitamin K1. Clinical and Applied Thrombosis/Hemostasis. 2016;24(1):5-12.
SOEDIRMAN J, DE BRUIJN E, MAES R, HANCK A, GRÜTER J. Pharmacokinetics and tolerance of intravenous and intramuscular phylloquinone(vitamin K1) mixed micelles formulation. British Journal of Clinical Pharmacology. 1996;41(6):517-523.
Koklu E, Taskale T, Koklu S, Ariguloglu E. Anaphylactic shock due to vitamin K in a newborn and review of literature. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;27(11):1180-1181.
Lulseged S. Haemorrhagic disease of the newborn: a review of 127 cases. Annals of Tropical Paediatrics. 1993;13(4):331-336.
Evidence on: The Vitamin K Shot in Newborns - Evidence Based Birth®. Evidence Based Birth®. 2019.
Haelle T. Here's The Truth About Vitamin K For Newborns. Forbes. 2016.
What is Vitamin K Deficiency Bleeding? | CDC. Centers for Disease Control and Prevention. 2019.
FAQs About Vitamin K Deficiency Bleeding | CDC. Centers for Disease Control and Prevention. 2019.
Imbrescia K, Moszczynski Z. Vitamin K. Ncbi.nlm.nih.gov. 2020.
DRI, dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academy Press; 2001.
VITAMIN K1- phytonadione injection, emulsion. Labeling.pfizer.com.
Iannelli V. Does the Vitamin K Shot Contain 100mcg of Aluminum? - VAXOPEDIA. VAXOPEDIA. 2018
Propylene Glycol. Atsdr.cdc.gov.
Iannelli V. That Black Box Warning on Vitamin K Shots - Keep Kids Healthy. Keep Kids Healthy. 2017.
Gropper S, Smith J. ADVANCED NUTRITION AND HUMAN METABOLISM; CENGAGE LEARNING CUSTOM P; 2012.
Stipanuk M, Caudill M. Biochemical, physiological and molecular aspects of human nutrition. 3rd ed. St. Louis, Mo: Elsevier; 2013.
Shearer M, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. Journal of Lipid Research. 2014;55(3):345-362.
Neil N, Malmfors T, Slovic P. Intuitive Toxicology: Expert and Lay Judgments of Chemical Risks. Toxicologic Pathology. 1994;22(2):198-201.
Scott S, Rozin P. Actually, natural is neutral. Nature Human Behaviour. 2020;4(10):989-990.
Black Box Warnings - Fast-Tracked Drugs & Increased Use. Drugwatch.com. 2020.